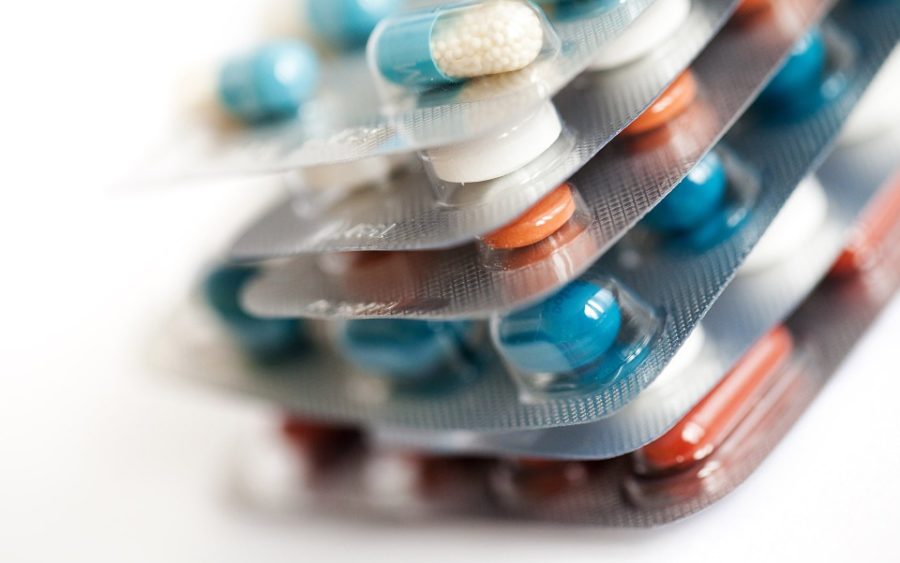
In 2011, the Falsified Medicines Directive was adopted with the aim of preventing the entry into the supply chain of falsified medicines. It amended Directive 2001/83/EC and it introduced harmonised European measures to fight medicine falsifications and ensure that medicines are safe and that the trade in medicines is rigorously controlled.
HOPE closely followed the EU legislative developments on this issue and replied to a public consultation on the concept paper on the detailed rules for a unique identifier for medicinal product for human use launched by the European Commission in April 2012 and participated to stakeholder workshops organised by the Commission representing the healthcare providers’ perspective. The Directive is in force since 2 January 2013.
In February 2016, a Delegated Act was adopted. The delegated act, applicable from February 2019, introduces medicine authentication by means of two safety features: a unique identifier and an anti‐tampering device, which will protect patients from the risks of falsified medicines and the consequences of common dispensing errors. It also provides for an end-to-end verification system to ensure authenticity and integrity of medicine packaging at dispensing points for patients, namely in pharmacies and hospitals.
With a view to facilitating compliance with the Regulation by 2019, HOPE conducted a mapping exercise of hospital representation within the National Medicines Verification Systems (NMVOs) in the Member States in 2016. Moreover, in February 2017 HOPE joined the European Medicines Verification Organisation (EMVO) as Associate Member. The EMVO is the not-for-profit organisation in charge of the medicines verification system management and governance created in February 2015. This collaboration will aim to facilitate a smooth implementation of the Regulation in European hospitals.
As of 9 February 2019, the Falsified Medicines Directive fully applied through the delegated act. From this date, the industry must affix a 2-D barcode and an anti-tampering device on the box of prescription medicines. The pharmacies – including on-line pharmacies – and hospitals have to check the authenticity of medicines before dispensing to patients.
Medicines produced before Saturday 9 February 2019 without safety features may also remain on the market until their expiry date. But the new end-to-end verification system will require authorised persons (and in particular pharmacists and hospitals) to verify, throughout the supply chain, the authenticity of the products.
On 8 February 2019, EMVO held a press conference in Brussels to mark the start of the Operational Phase of the European Medicines Verification System (EMVS) HOPE emphasised on that occasion that hospitals are making huge efforts to put all the requirements in place and listed the difficulties identified in several countries.
In September 2020, HOPE joined the European Association of Hospital Pharmacists in disseminated a questionnaire on aggregation so solve some of the issues identified in European hospitals.
On 13 January 2021, as a consequence of the Brexit, the European Commission adopted Delegated Regulation (EU) 2021/457 which amended article 22 of Delegated Regulation (EU) 2016/161 as regards a derogation from the obligation of wholesalers to decommission the unique identifier of products exported to the United Kingdom.